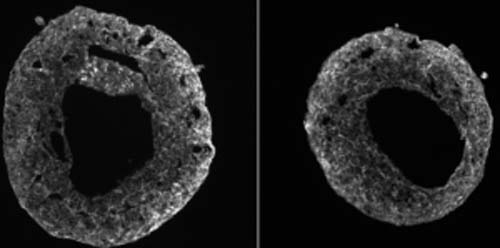
Self-organizing human heart organoids in a dish
Biologist Sasha Mendjan at the Austrian Academy of Sciences in Vienna and his team have used human pluripotent stem cells to grow sesame-seed-sized heart models, called cardioids, that spontaneously self-organize to develop a hollow chamber without the need of experimental scaffolds. This advance, which allows for the creation of some of the most realistic heart organoids to date, appears on May 20th in the journal Cell.
Previously, scientists have built 3D cardiac organoids via tissue engineering, an approach that generally involves assembling cells and scaffolds like building a house out of brick and mortar. But these engineered organoids do not have the same physiological responses to damages as human hearts and thus often fail to serve as good disease models.
“Tissue engineering is very useful for many things like, for example, if you want to do measurements on contraction,” says Mendjan. But in nature, the organs aren’t built this way. In the embryo, organs develop spontaneously through a process called self-organization. During development, the cellular building blocks interact with each other, moving around and changing shape as an organ’s structure emerges and grows.
“Self-organization is how nature makes snowflake crystals or birds behave in a flock. This is difficult to engineer because there seems to be no plan, but still something very ordered and robust comes out,” he says. “The self-organization of organs is much more dynamic, and a lot is going on that we do not understand. We think that this ‘hidden magic’ of development, the stuff we do not yet know about, is the reason why currently diseases are not modeled very well.”
Mendjan and his team wanted to mimic development by self-organization in a dish. They coaxed stem cells to self-organize by activating all six known signaling pathways involved in embryonic heart development in a specific order. As the cells differentiated, they started to form separate layers, similar to the structure of the heart wall. After one week of development, these organoids self-organized into a 3D structure that had an enclosed cavity, a similar spontaneous growth trajectory as human hearts. In addition, the team found the cardioids’ wall-like tissue contracted rhythmically to squeeze liquid around the inside the cavity.
“It’s not that we are using something different than other researchers, but we are just using all of the signals known,” Mendjan says. He adds that not all pathways are needed to direct stem cells to become heart cells. “So they thought, ‘Okay, they’re not really necessary in vitro.’ But it turns out all these pathways are necessary. They are important to make the cells self-organize into an organ.”
The team also tested how the cardioids respond to tissue damages. They used a cold steel rod to freeze parts of the mini-hearts and killed many cells at the site. Cell death is commonly observed after injuries such as a heart attack. Immediately, the team saw cardiac fibroblasts — a type of cell responsible for wound healing — start to migrate toward the injury sites and produce proteins to repair the damage.
“We want to come up with human heart models that develop more naturally and are therefore predictive of disease,” Mendjan says. “This way, companies will be more open to bringing more drugs into the clinical trials because they are much more certain of the outcome of the trial.”
The team has plans to grow cardiac organoids with multiple chambers like what’s seen in a real human heart. Many congenital heart diseases happen when other chambers start to form, so the multi-chamber model would help doctors better understand how defects develop in fetuses.
This work was funded by the Austrian Academy of Sciences (OEAW) and the Research Promotion Agency (FFG).
